Global Biological Indicator Vial Market to Reach USD 279.1 Million by 2036 Amid Rising Sterilization Standards
USA biological indicator vial market grow at 4.5%, driven by FDA rigor, advanced pharma infrastructure and demand for rapid, traceable sterilization validation.
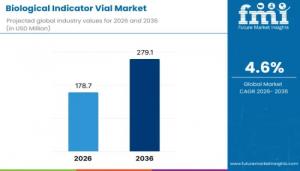
NEWARK, DE, UNITED STATES, January 21, 2026 /EINPresswire.com/ -- The global biological indicator vial market is valued at USD 178.7 million in 2026 and is projected to reach USD 279.1 million by 2036, expanding at a compound annual growth rate (CAGR) of 4.6% over the forecast period. This outlook reflects rising global emphasis on validated sterilization processes across healthcare, pharmaceutical manufacturing, food processing, dental practices, and laboratory environments.
Growth is being observed worldwide, with North America and Western Europe maintaining leadership due to stringent regulatory frameworks and mature healthcare infrastructure, while East Asia and South Asia Pacific are emerging as high-growth regions as pharmaceutical production scales and food safety enforcement strengthens. The market’s expansion is driven by the essential role biological indicator vials play in verifying sterilization effectiveness for steam, ethylene oxide (EtO), and hydrogen peroxide processes.
Discover Growth Opportunities in the Market – Get Your Sample Report Now
https://www.futuremarketinsights.com/reports/sample/rep-gb-6240
Market Context: Why Biological Indicator Vials Matter
Biological indicator vials are critical validation tools used to confirm sterilization lethality by detecting resistant microorganisms following sterilization cycles. Their use is mandated or strongly recommended under global quality and safety standards governing medical devices, pharmaceuticals, food production, and cosmetics.
Key demand drivers include:
• Rising procedural volumes in hospitals and surgical centers
• Expansion of sterile pharmaceutical manufacturing, including injectables and biologics
• Increased audits and inspection rigor across regulated industries
• Growth in contract sterilization services and reusable medical instruments
Together, these factors reinforce recurring demand for reliable, traceable sterilization validation solutions.
Quick Market Statistics
• Market Value (2026): USD 178.7 million
• Forecast Value (2036): USD 279.1 million
• Forecast CAGR (2026–2036): 4.6%
• Leading Sterilization Type: Steam sterilization
• Key Growth Regions: East Asia, South Asia Pacific, Western Europe
Segment Analysis Highlights
By Sterilization Type
• Steam sterilization indicators account for approximately 57% of global demand, supported by strong regulatory acceptance, compatibility with autoclave systems, and proven spore resistance.
• Ethylene oxide indicators represent 43%, serving low-temperature sterilization needs for heat-sensitive medical devices and specialized manufacturing environments.
By Incubation Time
• The 24 to 32 hours incubation segment holds the largest share at 45%, balancing reliability with operational efficiency.
• Up to 24 hours indicators account for 19%, reflecting increasing adoption of rapid-readout systems.
• Extended incubation segments (32–48 hours and above 48 hours) each hold 18%, supporting conservative validation protocols and legacy practices.
By End Use
• Food and beverages lead demand with 52% share, driven by strict food safety and thermal validation requirements.
• Pharmaceuticals represent 28%, reflecting sterile manufacturing expansion and regulatory oversight.
• Cosmetics account for 20%, emphasizing contamination control and batch verification.
Regional and Country-Level Growth Dynamics
Demand for biological indicator vials is rising across major manufacturing economies as validation expectations tighten and production capacity expands.
• China: CAGR 6.2%, driven by pharmaceutical expansion programs and food safety modernization
• India: CAGR 5.8%, supported by contract manufacturing growth and export certification requirements
• Germany: CAGR 5.3%, reflecting EU GMP compliance and advanced validation culture
• United States: CAGR 4.5%, underpinned by FDA oversight and rapid-readout adoption
• United Kingdom: CAGR 3.9%, supported by MHRA regulation and mature pharmaceutical production
• Japan: CAGR 3.5%, shaped by quality-driven manufacturing and disciplined validation practices
Technology and Workflow Trends Shaping Adoption
Manufacturers and end users increasingly prioritize:
• Rapid-readout biological indicators to reduce batch release delays
• Self-contained vial systems that minimize handling errors and contamination risk
• Enhanced traceability and documentation aligned with electronic quality management systems
These trends reflect the growing need to align sterility assurance with faster production workflows without compromising regulatory compliance.
Market Challenges and Constraints
Despite favorable growth prospects, the market faces several operational and regulatory challenges:
• Lengthy incubation cycles that delay production release
• High compliance and documentation requirements across jurisdictions
• Cost sensitivity among smaller facilities and emerging markets
• Capital investment and workflow changes required for rapid-readout adoption
Long-term scalability depends on continued innovation that shortens validation timelines while maintaining accuracy and regulatory alignment.
Competitive Landscape Overview
The biological indicator vial market is moderately consolidated, characterized by manufacturers with strong regulatory expertise, validated production capabilities, and established industry relationships.
Key players include:
• VWR Corporation (Avantor)
• STERIS plc
• Mesa Laboratories Inc.
• 3M Company
• Siltex Australia Pty Ltd.
• Getinge AB
• Terragene S.A.
• Crosstex International Inc.
Competition increasingly centers on rapid-readout technologies, self-contained designs, automated incubation systems, and digital documentation platforms. While traditional steam indicators face commoditization pressures, premium growth opportunities are emerging around workflow optimization, audit readiness, and lifecycle validation support.
Outlook Through 2036
As global industries strengthen sterilization validation requirements and regulatory scrutiny intensifies, biological indicator vials are expected to remain core components of sterility assurance strategies. Demand growth will be shaped by expanding pharmaceutical and food processing capacity, faster validation expectations, and continued emphasis on documented proof of compliance.
Why FMI: https://www.futuremarketinsights.com/why-fmi
Related Reports Insights from Future Market Insights (FMI)
Paper Core Market https://www.futuremarketinsights.com/reports/paper-cores-market
Masking Tape Market https://www.futuremarketinsights.com/reports/masking-tapes-market
Ampoule Filling and Sealing Machine Market https://www.futuremarketinsights.com/reports/ampoule-filling-and-sealing-machine-market
Linerless Label Market https://www.futuremarketinsights.com/reports/linerless-labels-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.